What type of ice melt should you use? What are the pros and cons? Which one damages concrete? I’ve compared all of them and presented the best ice melt based on your needs.
Calcium chloride melts the ice fastest, with minimal damage to the environment, but is also highly corrosive and damaging to concrete. Sodium chloride (salt) is the most common, but ineffective in low temperatures, corrosive to metals, and damaging to the environment.
As mentioned in my article on melting ice cubes, ice melts by absorbing heat, or energy.
When it’s cold outside, there is not much heat to absorb, so you need to use something to create a chemical reaction, to make it melt and prevent further water freezing. You can use active chemicals (including salt) to lower the freezing point of water. For the roads, we also use inert compounds (sand, ash) to add traction.
Let’s take a look as we examine all popular ice melts, and how they compare.
What Melts Ice?
To melt ice, you need to introduce foreign substances on ice, such as salt and other chemical particles that will make the ice melt faster. Most commonly used are rock salt, calcium chloride, magnesium chloride, and potassium chloride.
Ice melts fast when you mix it with something that lowers the freezing point of water, which chemical substances do.

As a homeowner, if you just want to melt the ice without any other additional benefits, then rock salt is fine. If however, you need to consider pets, children, and plant life then you should probably choose magnesium chloride or potassium chloride.
Companies use a combination of different chemicals, which usually works best. For example, the Ohio Department of Transportation regularly used a saline solution (brine) before cold temperatures occur, to prevent the formation of frost. Then, after brine (for the same winter), they used rock salt with liquid Calcium Chloride to better melt ice in extremely cold temperatures.
On top of that, they experimented with Ice Bite (formerly Geomelt) made from beet juice to increase the effectiveness of salt, so they didn’t have to use too much salt to prevent damaging the environment.
Mixing salt with other natural substances to increase the effect, and minimize the usage of salt seems to be the best strategy for melting the ice.
Ice Melt Materials
Ice melt materials generally fall into these two categories:
- Chemical – They create freezing-point depression, causing ice and snow to melt at lower temperatures:
- Sodium Chloride (table salt)
- Calcium chloride
- Magnesium chloride
- Potassium chloride
- Ammonium nitrate
- Ammonium sulfate
- Calcium Magnesium Acetate
- Potassium acetate
- Propylene glycol
- Urea
- Inert – These don’t generally melt ice much (if at all), but improve traction on ice; however, they can also ‘reduce’ traction when ice melts:
- sand
- brash, rubble
- slag
- wood ash
- sawdust
The first three chemicals are the most commonly used as deicers. We use inert ones mostly for traction for vehicles.
When choosing your own ice melt chemical, pay attention to its effect on vegetation, pets, and other animals (check below). Some of them can damage concrete, are corrosive to metals, and can sometimes (very rarely) damage pavement. Inert materials can sometimes damage vehicles and create dust.

What is Ice Melt?
The most basic ice melt material is salt, or more specifically, “rock salt”. It dissolves into liquid water and lowers its freezing point. This is basically how salts work on ice. It melts ice and prevents any future forming of ice provided enough salt is available. It’s the cheapest, but far from being the best.
The difference between rock salt and ice melt is in the mixture.
Ice melt is typically made as a combination of different chemicals, and/or acetate salts. They are usually more effective, pet friendly, and better for the environment than simply using rock salt.
Types of Ice Melt (commonly used)
Here are the most common types of ice melt:
- Sodium Chloride
- Calcium Chloride
- Magnesium Chloride
- Potassium Chloride
- Calcium Magnesium Acetate
- Potassium acetate
Sodium Chloride Ice Melt (salt)
Rock salt is the cheapest ice melt.
Sodium Chloride (salt) is one of the most used ice melts. It exists in various forms, such as rock salt, table salt, or as a concentrated solution mixed in water, called brine.
It is often used in the form of brine mixed with sand and gravel, but also with Calcium Chloride and/or magnesium chloride.
Sodium Chloride is effective down to 21°F (−6°C) and becomes ineffective below 14°F (−10°C). It is the most used type of ice melt because it is cheap and readily available in large quantities. It doesn’t last for long though, and you also need to be careful when using it next to plants and yard, because it can damage vegetation.
For those who were wondering, table salt does melt ice, but rock salt is commonly used.
Pros of Sodium Chloride Ice Melt
- inexpensive
- readily available in large quantities
- more effective than potassium chloride and urea
- minimal to no concrete damage (except rebar corrosion)
Cons of Sodium Chloride Ice Melt
- ineffective in lower temperatures
- highly corrosive to metals
- corrosive to rebar in concrete reinforcing
- harmful to soil and roadside vegetation
- harmful to aquatic plants and animals
- pollutes water and creates dangerous “chemical cocktails” which cause negative effects on drinking water supplies and ecosystems (research)
- causes problems in coastal coating applications
Sodium Chloride should be used with organic compounds (such as sugar beet, wood ash, ethanol, or calcium magnesium acetate) to increase the effectiveness of salt ice melt compounds, and reduce damage to the environment and metal structures due to high usage of salt.

To make sure salt doesn’t damage your plants, yard, and infrastructure, you can use Epsom salt (which is actually magnesium sulfate), or mix your salt with beet juice, glycol, sugar, and Calcium Chloride, or magnesium chloride.
Calcium Chloride Ice Melt
Calcium Chloride is the most effective ice melt.
When Sodium Chloride becomes ineffective in lower temperatures, we can use Calcium Chloride. It is most effective in temperatures lower than 21°F (−6°C), down to -20°F (-29°C).
Even though it’s much more effective than Sodium Chloride, it also costs about 6 times as much, and it is corrosive to metals such as iron, copper, and aluminum. This can be a problem because metals exist almost everywhere: in concrete, container materials, infrastructure, vehicles, equipment, and in lots of other places.
Generally, non-chloride deicers are less corrosive than chloride-based deicers.
Pros of Calcium Chloride Ice Melt
- Much more effective than other common deicers [research]
- Works on lower temperatures than most other ice melts
- Works faster than any other ice melt
- Less harmful to plants and soil
Cons of Calcium Chloride Ice melt
- Corrosive to metals (in concrete, vehicles, equipment, etc.)
- Damages concrete twice as fast as magnesium chloride
- Can damage aquatic life and water if overused
- Slightly damaging to soil and vegetation
- Can be expensive (6 times as much as Sodium Chloride)
Magnesium Chloride Ice Melt
Magnesium Chloride is good for the environment.
Magnesium Chloride doesn’t corrode surfaces as much, is less damaging to concrete, less toxic, and environmentally safer than Calcium Chloride and Sodium Chloride. Magnesium chloride is also pet-friendly but may cause stomach upset.
You can purchase it as flakes, pellets, or a liquid.
You can use it for low-temperature de-icing for highways, sidewalks, and parking lots, and has a practical melting temperature of -10°F (or -23°C).
You may want to use magnesium chloride in a combination with sodium chloride to improve deicing performance while reducing the need for damaging salts in sodium chloride.
Magnesium chloride is used in 3 ways:
- Anti-icing – spreading onto the roads before snow storm to prevent snow from sticking and ice from forming
- Prewetting – sprayed directly onto salt so the salt sticks on the road
- Pretreating – mixed with salt and spread onto paved roads
Pros of Magnesium Chloride Ice Melt
- Lower melting temperature than sodium chloride
- Less damaging to concrete than calcium chloride
- Less toxic to plants and animals
- Environmentally safer than both sodium chloride and calcium chloride
- Can be used for anti-icing, prewetting and pretreating
Cons of Magnesium Chloride Ice Melt
- Highly corrosive to metals (twice as much as calcium chloride)
- Slightly damaging to concrete
- Highly damaging to concrete reinforcing (corrosive to rebar in concrete)
- Will contaminate water and aquatic life if overused
- Spraying can cause slight foliage damage and harm roots
Is Magnesium Chloride Safe?
Magnesium Chloride is ‘safer’ than Sodium Chloride and Calcium Chloride but is not completely safe. It is safer for soil and vegetation, less irritating on the skin, corrodes metal less, and is twice as much safer for concrete than calcium chloride.
Potassium Chloride Ice Melt
Potassium chloride is best for the environment.
Potassium chloride ice melt is not the most effective ice melt, but it is environmentally friendly. It costs about the same as calcium chloride and is not chemically damaging to concrete.
In the US, we don’t often use it for deicing as much as other ice melts, and Potassium acetate is more commonly used.
Pros of Potassium Chloride Ice Melt
- Works on lower temperatures down to 12°F (-11°C)
- Not damaging to concrete
- Nearly harmless to plants and animals
Cons of Potassium Chloride Ice Melt
- Works slower than calcium chloride, rock salt and magnesium chloride
- Moderately corrosive to metals
- More expensive than sodium chloride (about the same as calcium chloride)
Potassium Acetate Ice Melt
Potassium acetate ice melt is a substitute for chloride salts and offers the advantage of being less aggressive on soils and much less corrosive, however, it is also much more expensive. It is mostly used in airports and airfields.
Pros of Potassium Acetate Ice Melt
- less damaging on soils
- much less corrosive
Cons of Potassium Acetate Ice Melt
- much more expensive
- can be damaging to concrete
Best Liquid Ice Melt
The best liquid ice melts are Potassium Acetate and Calcium Chloride (salt). Liquid ice melts are typically used for pre-wetting road salt, sand, or other solid deicers, or are mixed with salt brine as a liquid deicer. Compared to 7 other liquid ice melts, potassium liquid and calcium chloride liquid are the fastest in melting ice.
Research on 9 different liquid deicers has shown that higher concentrations of liquid ice melt produced higher amount of ice melting at 0°F, 10°F and 20°F or -6.7°C, -12.2°C and -17.7°C.
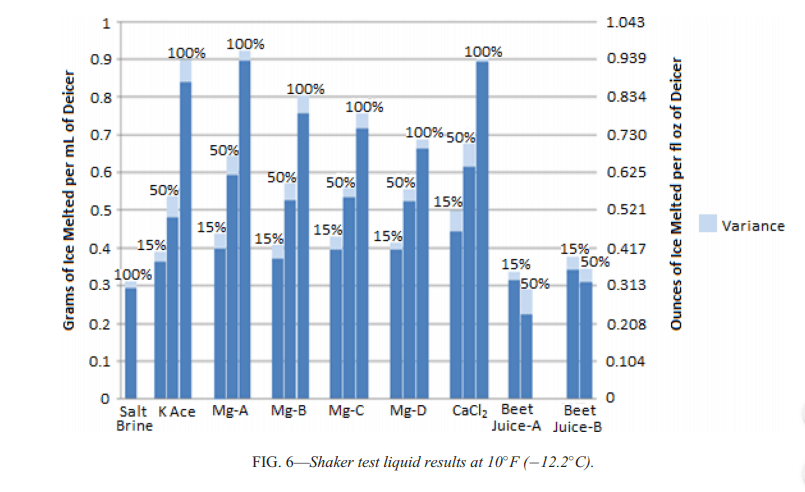
Salt brine and beet juice were ineffective in melting ice at 0°F, while liquid sodium chloride consistently performed worst as an ice melt, except when mixed at 50/50 with beet juice.
Best Ice Melt for Asphalt
The best ice melt for asphalt is calcium chloride. It is the most effective ice melt in low temperatures, works the fastest, and is minimally damaging to the environment. It is corrosive and highly damaging to concrete, but chloride-based ice melts aren’t typically damaging to asphalt.
According to research, chloride-based deicers (salt and other chlorides) had little damaging influence on the asphalt pavement, since asphalt binder is chemically resistant to these chloride-based ice melts. The only thing you need to worry about is the effect on the environment.
If your asphalt is new, check out my article on how long for asphalt to dry. Chlorides affect both new and old asphalt just the same.
What is Concrete Safe Ice Melt?
Sodium chloride and potassium chloride are the least damaging to concrete. On the other hand, calcium chloride is the most damaging to concrete.
While comparing three chloride-based ice melts (Sodium Chloride, Calcium Chloride, and Magnesium Chloride) research showed that Calcium Chloride (salt) was the most damaging to pavement concretes when used as a deicing solution, being twice as damaging as magnesium chloride.
Sodium Chloride showed almost no damage, while magnesium chloride was only slightly damaging (even though the image might imply differently).
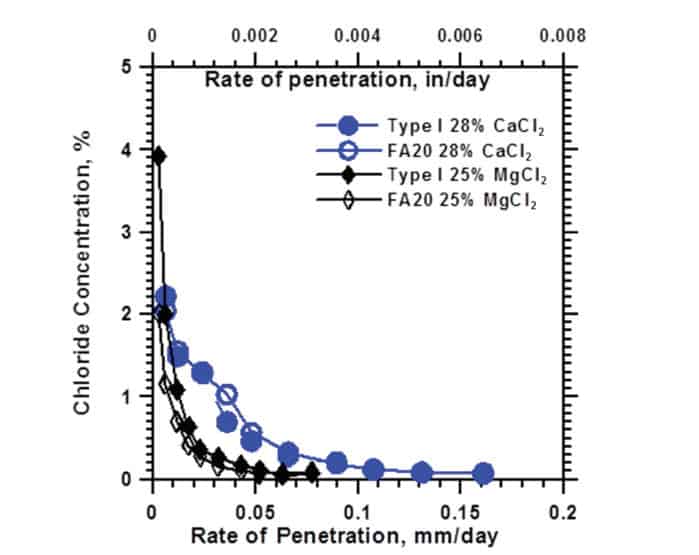




Note: Magnesium chloride is twice as less damaging to concrete than calcium chloride, even though it may appear different in pictures.
Another research from the Utah Transportation Commission showed similar results: Sodium Chloride (salt) wasn’t damaging, while Calcium Chloride and magnesium chloride were significantly damaging the concrete causing cracking, mass loss, and strength loss.
Best Ice Melt for Concrete
Out of chlorides, the best ice melt for concrete is Sodium Chloride, together with Potassium Chloride. Sodium Chloride (salt) does almost no damage to concrete, except in concrete reinforcing, where it will initiate the corrosion of rebar. [1], [2], [3], [4], [5]
Potassium chloride is also not chemically damaging to concrete. In fact, potassium chloride increases the comprehensive and tensile strengths of OPC, PBC, and BCC concrete when casting concrete blocks. The only damage to concrete can be from freeze-induced expansion pressures by increasing the number of freeze/thaw cycles.
Another study from Kansas University showed calcium magnesium acetate to be highly damaging to concrete as well.
This would also apply to ice melt for new concrete, where sodium chloride and potassium chloride seem the best, while calcium chlorides and acetates seem the worst of ice melts.
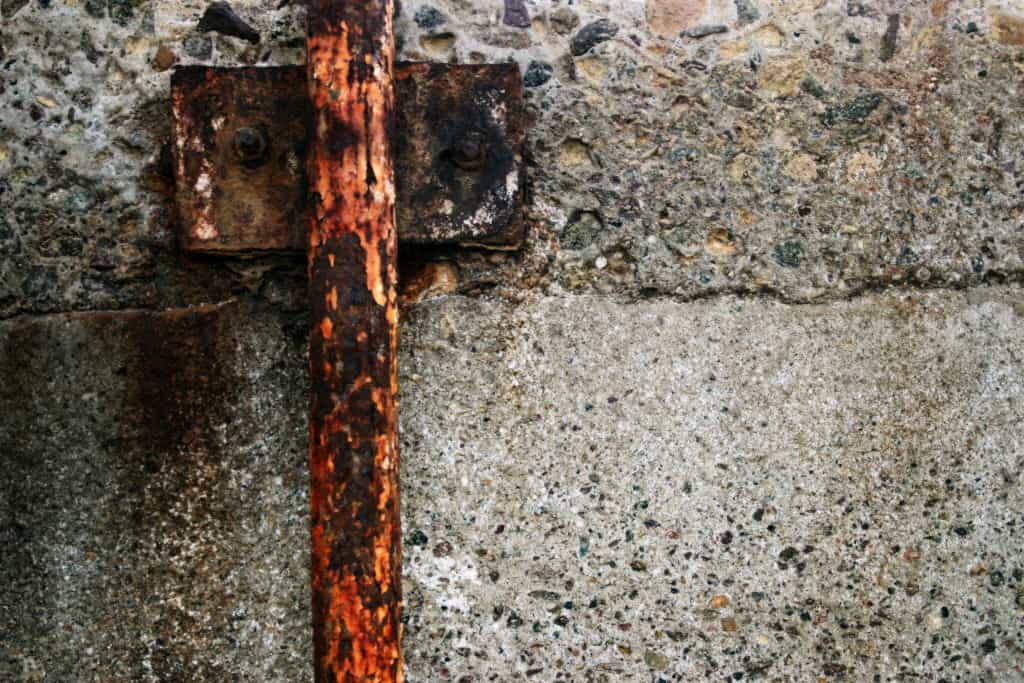
How to Reduce Corrosion from Ice Melt?
Chloride-based deicers such as Sodium Chloride can corrode rebar in concrete, metal in infrastructure, vehicles, and any other type of equipment made out of metal. To prevent corrosion we can still use chloride-based ice melts, but strategically, to minimize the usage and increase the effectiveness.
Here is how to reduce corrosion from ice melt:
- Limit the amount of chloride-based ice melts
- only use when required (when temperatures are very low)
- reduce accumulation of snow and ice through snow fences
- distribute the usage of ice melts better
- use pre-wetting – jumpstarts the melting and increases the effect
- Combine chloride-based deicers with each other, or with other liquids such as beet juice, to increase efficiency, and reduce the usage of salts
- Thoroughly wash vehicles and equipment after direct exposure to chloride deicers or to brine
Since chloride-based ice melts are corrosive to metals, in airports, for example, other substances are used:
- sodium formate
- potassium formate
- sodium acetate
- potassium acetate
How to Melt Ice Fast Without Salt?
There are many ways to melt the ice without using salt. The only difference from melting with salt is that it may not be as fast as you’d imagine, depending on what you use.
Other ice melts besides salt are:
- beet juice
- sugar
- rubbing alcohol
- fertilizer/compost (don’t use too much)
- wood ash
- vinegar
DIY Ice Melt (Homemade Ice Melts)
There are many ways to make your own de-icer, so you can simply use a product from your house directly to melt ice.

Here are the products that you can use as a homemade de-icer:
- Sugar. Similar to salt, sugar can be used to de-ice, and works in the similar way to table salt – lowering the freezing point of water.
- Beet juice. Lowers the freezing point of water by itself, or combined with salt brine. The Ohio Department of Transportation used Ice Bite (former Geomelt) product with beet juice and salt for ice melt, and determined it works well at minus 20°F.
- Compost/Fertilizer. If you’ve used the grass from your yard to create a compost, you can use it as a deicer. Compost usually has ammonium sulfate, potassium chloride or urea, which will melt the ice. Not as effective as salt, but still useful. NOTE: careful with fertilizer – using too much can damage your property and waterways.
- Wood ash. Contains potash, or potassium salts, which melts the ice and adds traction on the roads. You can use fireplace ash. Wood ash is black in color and absorbs heat from the sun, which will melt the ice even faster on the sun. NOTE: Some wood ash can be harmful to plants.
- Kitty litter, sand, gravel, sawdust. These are well known traditional deicers for roads, which don’t help as much with melting as they do with adding traction, and are used in combination with ice melt.
- Alfalfa meal. Rich in nitrogen and adds traction, but might produce alfalfa sprouts in the springtime.
- DIY ice melt #1:
- 2 quarts of warm water
- 2 ounces of rubbing alcohol
- 6 drops of dish soap
- mix together and put either into spray bottle or larger canister
- DIY ice melt #2:
- Since vinegar helps with melting ice, you can use it as deicer
- Mix 60% vinegar and 40% of water to create a mixture.
- Pour over the ice to melt.
- Shovel away the snow that melted to prevent it turning into hard ice.

Pet Safe Ice Melt
To make sure you are using pet-safe ice melt, you can choose 100% organic ice melts, which are usually all-natural and salt-free.
Salt can cause gastrointestinal problems to pets if eaten, but luckily, there are many products without salts, which can safely be used.
Some of the best pet-safe ice melt products are:
Safe Paw Ice Melt
Safe Paw is an excellent all-natural ice melt product, completely safe for pets. Not only is it safe for pets, but also non-corrosive as well. It is composed of a crystalline amide core and combined with friendly glycols.
We previously saw that propylene glycol is sometimes used as an ice melt, but also in various edible items such as coffee-based drinks, liquid sweeteners, ice cream, and other products. This means glycol, found in Safe Paw, is completely safe for your pet.
Safe Paw ice melt pros:
- 100% pet safe
- 100% kid safe
- no salt (no corrosion on metals or concrete)
- chlorine free (no health issues)
- environmentally safe
- works on -15°F
On their website, you can find lots of results and reports, such as test results, as well as toxicology reports, approval, specification, and chemical composition testing.
Safe Step Ice Melt
Safe Step “Sure Paws” is another great all-natural ice melt, which is safe for pets plus less damaging on concrete and vegetation. It is pet-friendly, melts ice down to -15°F (-26°C), and is not aggressive on concrete and vegetation when used in moderation.
Stay safe!
Credits: cover photo By Z22 – Own work, CC BY-SA 3.0









![How Much Do Shipping Containers Cost? [Full Guide]](https://howmonk.com/wp-content/uploads/2020/03/Shipping-Container-retail-shop-230x200.jpg)

