Ice melts by absorbing heat or energy. When we put an ice cube in the living room, it will begin to absorb the heat and melt. How long will this take for an average-sized ice cube?
A 1-inch ice cube at 75°F room temperature (24°C) will take 45 to 60 minutes to melt. A standard 1-ounce cube (30 grams) will take 90 to 120 minutes to melt at the same temperature. The same 1oz (30g) ice cube submerged in a cup of hot water at 185° F (85° C) will take about 60-70 seconds to melt.
Remember that these are just averages and approximations, and how long it will take depends on many factors listed below. On average, an ice cube can take anywhere from 30 minutes to 2 hours to fully melt.
Factors determining how fast the ice cube melts
The main factors that determine how fast the ice cube melts include the following:
- The temperature of the ice. The warmer the temperature of the ice cube, the faster it will melt.
- Air temperature of the room (or water if submerged in a liquid). Higher air or water temperature will speed up the melting of the ice cube.
- Mass, shape, and volume of the ice. Larger and thicker ice cubes will take longer to melt than smaller and thinner ones. Similarly, a cube shape has more surface area exposed to the air so it will melt faster than a sphere of the same volume.
- Amount of air volume in the room: More air in the room means more heat exchange, thus accelerating the melting of the ice cube.
- Insulation factors (material that the ice cube is placed on). Different materials conduct heat differently. For example, metal conducts heat much better than plastic, so if the ice cube is placed on metal, it will melt faster than plastic.
How Long Does It Take for an Ice Cube to Melt in Cold Water?
The time it takes for an ice cube to melt in cold water depends on several factors, including the size of the ice cube, the temperature of the water, and the amount of agitation in the water.
On average, a 1-inch ice cube will take 15-30 minutes to melt in cold water fully and 5-10 minutes in room temperature water.

The speed of an ice cube melting in water depends mostly on these three factors:
- The size of the ice cube. Larger ice cubes will take longer to melt than smaller ones, and more irregularly shaped ice cubes will melt more quickly than those with a uniform shape, as they have more surface area exposed to the water.
- The starting temperature of the water. The colder the water, the longer it will take for the ice cube to melt. Warmer water will speed up the melting process.
- The surrounding air temperature. Warmer air will cause the ice cube to melt more quickly as it will transfer heat to the water, while colder air will slow down the melting process.
- Stirring or agitation of the water. Stirring or agitating the water can speed up the melting process by increasing the surface area of the ice cube exposed to the water and promoting heat transfer.
- Presence of dissolved substances in the water, such as salt or sugar. Adding salt or sugar to the water will increase its freezing point, meaning it will take longer for the ice cube to melt.
- The type of container holding the ice and water. Some materials are better conductors of heat than others, and the type of container holding the ice and water can affect the melting speed by either speeding up or slowing down the transfer of heat from the surrounding environment to the ice cube.
- The amount of exposure to sunlight or other heat sources. Direct exposure to sunlight or other heat sources can increase the melting speed of the ice cube.
Ice melts when heat energy causes the molecules to move faster. In this melting process, the water molecules absorb energy. The faster the absorption, the faster the ice cube melts.
How Long Does It Take for an Ice Cube to Melt in Boiling Water?
An ice cube will melt almost instantly in boiling water, usually taking less than a minute to fully dissolve. The high temperature of boiling water greatly accelerates the melting process compared to cold or room-temperature water.
When an ice cube is placed in boiling water, it will melt much faster than in cold or room-temperature water. This is because boiling water has a temperature of 100°C (212°F), which is well above the melting point of ice, which is 0°C (32°F). When the temperature of the water is so much higher than the melting point of ice, the heat is rapidly transferred from the water to the ice, causing it to melt quickly.

How Many Ice Cubes Are Needed to Cool Down Water?
The exact amount of ice cubes needed to cool down a specific amount of water to a specific temperature can vary greatly depending on several factors, such as the size and shape of the ice cubes, the starting temperature of the water, and many others.
However, we have tested this and concluded that to cool down 6.76oz (200ml) of water at 194°F (90°C) to 50°F (10°C), we would need 6 regular-sized ice cubes of 1oz (30g).
To quickly cool down a cup of 16oz (470ml) of water (or tea) from 185°F (85°C) to 122°F (50°C), we need about 4 regular-sized ice cubes of 1oz (30 grams).
This is the number of ice cubes necessary to cool down water to a given temperature, meaning this amount is sufficient.
Ice Cube Melting Experiment
There are many different experiments that you can do with melting ice cubes. Here’s a simple experiment you can do to answer the question: Which shape of an ice cube will melt the slowest?
- Get a few different ice trays (cube, spherical and rectangle-shaped)
- Freeze them up with the same amount of water
- Take the cubes out and put them into 3 identical plastic cups
- Measure how much time it takes for the ice cubes to melt
Conclusion: Which ice cube melts the slowest? The larger the surface area of the ice cube, the more heat it will absorb, meaning the spherical-shaped cube will melt the slowest.
Why? Because the less surface area, the less heat can be absorbed by the ice and, therefore, will melt less than the other types.
Which Ice Cube Will Melt the Fastest
If we take two very similar ice cubes with the same density and temperature, the ice cube with the largest surface area will melt the fastest.
Many good ice cube makers can make some nice looking ice cubes of various sizes and surface areas, like this one for whiskey and cocktail, or this one that makes crystal-clear ice cubes. Try one and test for yourself.
These cool factors determine when one ice cube melt faster than the other:
- the temperature of the ice cube
Ice cubes can be chilled even below 32 degrees F, which is the temperature of the water freezing. The colder the ice, the slower it will melt. To have the ice melt faster, you would want close to 32F.
- Adding salt or sugar on ice cube will make it melt faster
Introducing foreign substances on ice, such as salt or chemical particles, will melt ice cubes faster. The ice cube with salt melts faster because the air around it is warmer than 32°F.
When you add salt, it dissolves into the water of the ice cube. Saltwater freezes at a lower temperature than freshwater, which is why salt is used to melt ice on sidewalks and roadways.
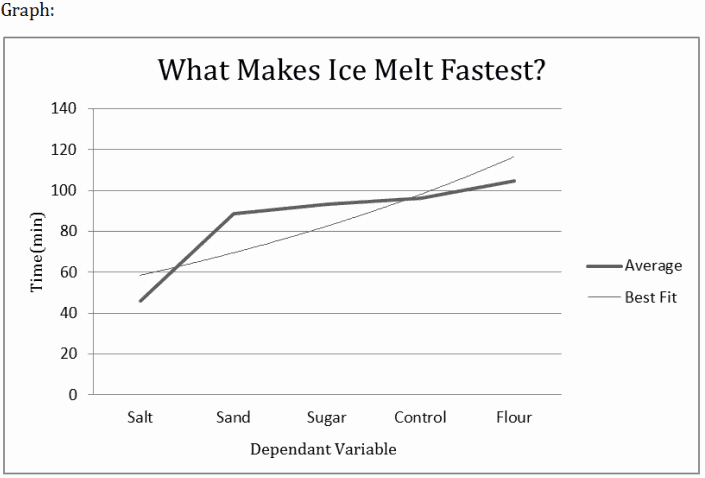
Here we can see how other substances affect the melting of ice, where the salt melts it the fastest out of these five.
However, as shown in my blog post on the best ice melt, other substances can help with melting an ice cube, such as calcium chloride, magnesium chloride, or glycol. More in this blog post on ice melts.
- putting ice cube in a larger cup of hot water will cause it to melt faster
If you were to try to melt ice in a cup of hot water, it would melt faster the bigger the cup is. This is because there is a larger volume of hot water to absorb energy. If you were to put it a large block in a cup, it would cool down the water and be left with very little energy to absorb. As we know, cold water has less energy than warm water.
- putting a large block of ice in a large room will make it melt faster than if you put it in a smaller room (or a smaller container)
This is similar to the previous example: there will be a lot more hot air with energy that could be transferred onto the ice, which will cause it to melt faster.
Related Questions:
How Long Does It Take for a Bag of Ice to Melt?
A 3lbs (1.5kg) bag of ice will melt at a room temperature of 73°F (23°C) in approximately 5-7 hours. This will depend on many factors, such as outside temperature, the ice’s shape, and whether the ice is inside or outside the bag.
How Long Does It Take a Gallon of Ice to Melt?
If the ice is inside a gallon container at room temperature, it will take approximately 12-15h or longer. If it’s outside of the container, it will take about 6-8 hours, depending on a few factors.
How long does it take for an ice cube to melt in salt water?
The time it takes for an ice cube to melt in salt water can vary depending on several factors, such as the concentration of salt in the water, the temperature of the water, the size and shape of the ice cube, and the presence of other materials that may impact the rate of melting. On average, it may take a few minutes to several hours for an ice cube to fully melt in salt water.
How long does it take for a block of ice to melt?
The time it takes for a block of ice to melt depends on several factors, including the size and shape of the block, the temperature of the surrounding environment, and the presence of any additional heat sources. On average, it can take several hours for a large block of ice to melt fully in a room-temperature environment.
Does ice melt faster in tap water or saltwater?
Ice typically melts faster in saltwater than in tap water. Saltwater has a lower freezing point than tap water, so it takes less energy to melt the ice. The salt in the water causes the ice to melt by lowering the freezing point of the water and causing it to absorb heat more quickly.
Does blowing on ice speed up melting?
Blowing on ice can increase the melting rate because it increases the air movement around the ice, which can cause it to lose heat more quickly.
Does ice melt from the top down or the bottom up?
Ice melts from the top down and the bottom up. The bottom of the ice cube is in direct contact with the warmer surface and begins to melt, while the top surface is exposed to air and starts to lose heat through evaporation, causing it to melt as well.
How Much Does an Ice Cube Weigh?
A typical ice cube from a standard ice tray weighs about 1 ounce, or 30 grams (28g more precisely, because of the density of ice). A smaller 1 inch ice cube weighs about 0.53oz (15g).





![How Much Do Shipping Containers Cost? [Full Guide] Shipping Container retail shop (cost)](https://howmonk.com/wp-content/uploads/2020/03/Shipping-Container-retail-shop-300x200.jpg)






